1 Preparing broodstock fish for spawning
Obtaining broodstock
1. To propagate your fish successfully, you need healthy and sexually mature individuals of both sexes. These will be your broodstock.
2. There are two ways to obtain such broodstock.
(a) Fish may be captured from natural waters with fishing gear (see Sections 8.2 and 8.3) and transported alive to your fish farm (see Chapter 13). There they are stocked either in broodstock ponds until they become sexually mature, or if they have been captured during their spawning season and are already mature, in storage ponds.
(b) Fish breeders can be raised on the farm itself, which makes it easy to improve your stock progressively through careful management.
Managing broodstock ponds
3. The farm ponds where you plan to raise your broodstock should be well adapted to the fish species. Well-regulated temperatures and well- oxygenated water (see Sections 2.4 and 2.5) will contribute to successful rearing. There should be an ample supply of natural food available (see Section 10.1), well adapted to the particular feeding regime of the fish breeders. During the maturation period, protein-rich food ingredients (see Section 10.2) should be supplemented if necessary. Adapt the stocking density of the breeders to the food supply available but keep it, in all cases, relatively low. Select your broodstock ponds so that they are easily accessible but secure from poaching.
4. It is best to keep a relatively young broodstock made of small to medium- sized fish, depending on the species. Small breeders of common carp and Chinese and Indian carps weigh from 0.5 to 2 kg, while medium breeders weigh from 2 to 5 kg. Small breeders of tilapias weigh from 0.15 to 0.25 kg, while medium breeders weigh from 0.25 to 0.40 kg. Such fish produce more eggs of a better quality, with a better food utilization efficiency.
Selecting suitable breeders
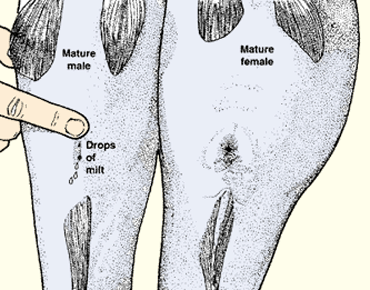
5. When the breeding season comes, broodstock should be carefully selected. Only fish that are ready to spawn should be used. Select fish with the following characteristics.
(a) Males should release a few drops of milt when the abdomen is slightly pressed.
(b) Females should have a swollen and protruding genital opening, reddish/rose in colour, and a well-rounded and soft abdomen, showing that the gonads are developed up to the dormant stage.
 |
6. When there is risk of male aggression (e.g. in the case of catfish) or uncontrolled spawning (e.g. tilapias and common carp), the two sexes should be kept in separate ponds after selecting them.
Using pituitary glands to propagate fish
7. If you wish to propagate your fish semi-artificially or artificially, you need a supply of the chemicals (or hormones*) which play a decisive role in ovulation, the final ripening of the dormant eggs. These chemicals, the gonadotropins*, are produced, accumulated and stored in the pituitary gland of the fish, also called hypophysis*, while they become sexually mature.
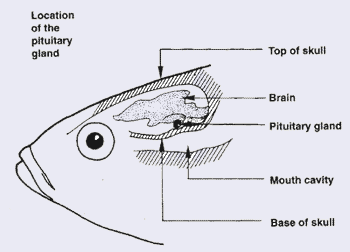
8. This small pituitary gland can be found in the upper part of the fish head, on the ventral side of the brain. It is quite easy to collect such glands from mature fish, store them for later use if necessary and extract the gonadotropin hormones from them, as you will learn in the next paragraphs.
9. It is very important to collect pituitary glands from suitable fish, to be certain that these glands contain enough gonadotropins to be effective. Select fish with the following characteristics:
- sexually mature;
- preferably alive or freshly killed;
- suitable size.
Note: most pituitary glands collected commercially belong to large fish such as common carp and salmon. They may be used for the propagation of other species also.
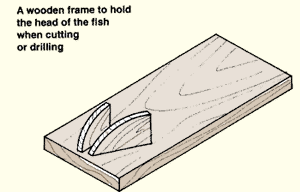
10. The pituitary gland can be collected from a freshly killed fish in two ways: by cutting open the head or by removing the pituitary gland with a drill. It is easier to work on the head of a fish if you have a wooden frame to hold it firmly in place when cutting or drilling.
 |
 |
Collecting pituitary glands by cutting open the head
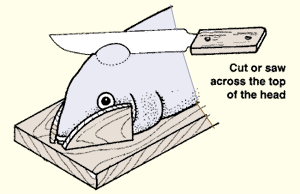
11. To cut open the head, proceed as follows.
| (a) Remove the top part of the skull with a saw or a strong sharp knife. | (b) Locate the pituitary gland in the brain mass. (c) Remove it carefully with forceps. | |
 | 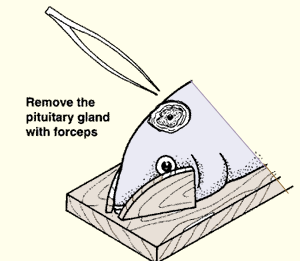 |
Collecting pituitary glands by drilling into the head
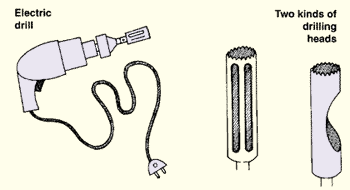 |
12. It is often easier to use a drill, preferably an electric one, and a special drilling head which you can have made in a local workshop. Proceed as follows.
| (a) Select or make a drilling head that is the correct size for you (see note in para. 13). (b) Locate the drilling point on the top point of the skull, as shown. | (c) At the drilling point, press a wooden guide piece (with a hole drilled) against the skull. | |
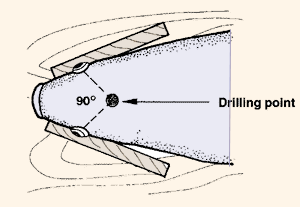 | ||
| (d) Drill through the top of the skull, the brains and the base of the skull, down to the mouth cavity. |
(e) Take out the drilling head together with the small plug of bone and tissue it contains.
(f) Remove this plug of material from the drilling head.
(g) Cut off the upper half of this plug and carefully separate the brain tissue lying at the base of the skull from the lower half.
(h) Pick out the pituitary gland from this with forceps.
13. You may either use this gland immediately or store it for later use (see paragraph 14 on).
Note: to ensure that the gland comes with the drilled core, select the diameter of the drilling head according to the size of the fish: appropriate diameters are 2.5 cm for fish up to 1 kg, 4 cm for fish up to 3 to 4 kg, and 5 to 6 cm for larger fish.
Storing fresh pituitary glands
14. When you plan to keep the pituitary glands for a certain time, a simple way to treat them is as follows.
| (a) As you collect the fresh gland from the fish, place it in a small bottle containing acetone. This chemical will start removing water and fat from the gland. It will harden and preserve the gland and the hormones it contains. | (b) Collect together, in the same bottle, all glands obtained on the same day. | |
(c) About every eight hours replace this acetone with new acetone, over a total period of 24 hours. Then, drain out all acetone.
(d) Dry the hardened glands on blotting paper.
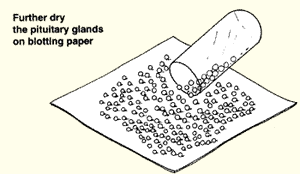 |
(e) Put the dry glands in small glass containers and press them down with a ball of fine cotton wool. Cork the container tightly and seal it with wax or other sealing material such as paraffin. Label it, indicating the origin of the glands and the date of their collection.
(f) Keep these sealed containers either in a plastic bag or in a desiccator or air tight storage jar, in the presence of a desiccating chemical such as silica gel or calcium chloride.
15. Acetone-dried pituitary glands can be safely stored this way for several years, without the need for refrigeration, as long as they are kept free from moisture. You may also keep fresh glands in a freezer.
Extracting gonadotropic hormones from pituitary glands
16. The gonadotropic hormones which are injected to induce ovulation and/or spawning are extracted from the pituitary glands, either immediately on collection or after a certain storage period. Proceed as follows.
(a) Obtain the number of glands required on the basis of the dose of hormones to be used according to specialized manuals.
Example
You are planning to use dried pituitary glands with 34 females (average weight 2 kg; two injections each) and 17 males (average weight 1.5 kg; one injection each); you will require the following quantities of dried glands:
| 34 fish x 2 kg x 0.3 mg/kg = | 20.4 mg | ||
| 34 fish x 2 kg x 3.5 mg/kg = | 238.0 mg | ||
| 17 fish x 1.5 kg x 2.0 mg/kg = | 51.0 mg | ||
| 309.4 mg | |||
| 31.0 mg | |||
| 340.0 mg |
If the average weight of one gland is 2.5 mg, you will require 340 mg � 2.5 mg = 136 pituitary glands.
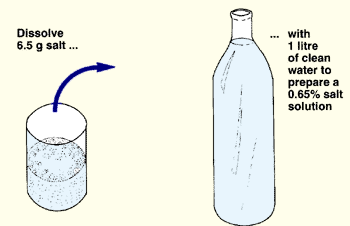
(b) Prepare a 0.65 percent salt solution (saline): dissolve 6.5 g of common kitchen salt in 1 litre of clean water. You may use either boiled and filtered water or distilled water. Use clean glassware and mix well. Keep this solution in a corked glass bottle.
 |
(c) Calculate how much salt solution you require, according to the manuals referred to above.
Example
For the above example, you may require:
- females, first injection 1 ml/fish: 34 x 1.0 ml = 34 ml
- females, second injection 1.5 ml/fish: 34 x 1.5 ml = 51 ml
- males, one injection 1.5 ml/fish: 17 x 1.5 ml = 26 ml
Add 10 percent to these calculated volumes of salt solution to compensate for losses.
(d) Grind the required number of glands into a mash or fine powder in a mortar.
Example
For the first series of injections, you require 20.4 mg + 10 percent = 20.4 mg + 2.1 mg = 22.5 mg of glands,
or about 22.5 mg � 2.5 mg = 9 glands to be finely ground.
(e) Measure the required volume of 0.65 percent salt solution and pour it in the mortar, over the gland mash/powder. It is best to use a syringe to measure such small volumes.
Example
For the first series of injections, measure 34 ml + 10 percent = 34 ml + 3.4 ml = 37.4 ml of the prepared salt solution.
(f) Mix the saline and gland material well to extract the gonadotropic hormones from the gland tissue into the liquid.
(g) Let it settle, or better still, use a small hand centrifuge to separate the upper (supernatant) liquid from the pieces of gland material.
How to give the hormonal injection to fish
17. Selected breeders should be prepared for hormonal injection. Use the following procedure.
(a) Early in the morning, select from the storage ponds the male and female breeders to be injected that day. Check carefully that they are ready to spawn, as described in paragraph 5.
(b) Bring them close to the spawning area or into the hatchery. Store them in a net or a tank, in a good water supply, keeping the sexes separated.
(c) Prepare all the necessary materials: suitable quantites of hormone extract, clean working area, recovery tank, pond or net for the broodstock. Once these are ready, prepare the fish.
(d) If possible, anaesthetize the fish to be injected in 50 to 100 l of a suitable chemical solution (see Section 8.7); if the individual size of your fish is not uniform, treat them successively in batches of similar sized fish.
(e) At all times, watch the sedated fish closely and, if necessary, transfer them back to well-aerated water quickly.
18. Then proceed with the injection of the hormonal extract.
(a) According to the live weight of each breeder, fill a syringe with the exact dose of pituitary gland extract necessary.
(b) Take the breeder out of the water with a dip net (see Section 8.4). Place the fish gently over a soft smooth surface such as a piece of rubber foam and immobilize it inside the net.
(c) Slowly give the injection at a 45� angle as follows:
- if the fish is not scaly, such as mirror or leather common carps, give an intramuscular injection either below the tip of the dorsal fin or in the caudal peduncle, using the pressure of a finger to avoid spilling out the hormonal extract;
- if the fish is scaly such as Chinese and Indian carps, give the injection into the body cavity, either behind the base of the abdominal fin or under the base of the pectoral fin, being careful to insert the needle beneath the scales and not through them.
19. Immediately after the injection, replace the fish in well-aerated water. Depending on the propagation method used, the fish may be:
- either replaced in the storing net or tank where the initial development of the dormant eggs takes place; a second dose of hormones is then given to the fish for the eggs to reach the final ripening stage during the ovulation period;
- or placed in the area prepared for receiving a certain number of males and females; spawning takes place usually a few hours later.
Example
Proceed as follows for the following types of fish:
- common carp at 24�C: preparatory injection (females only); + 12 hours make decisive injection (females and males);
+ 10 hours = induced spawning or artificial propagation; - Indian carp at 30�C: preparatory injection (females only); + 6 hours make decisive injection (females and males);
+ 3 to 6 hours = induced spawning; - Chinese carp at 24�C: preparatory injection (females only); + 18 to 24 hours make decisive injection (females and males);
+ 9 hours = induced spawning or artificial propagation.
Ripening eggs
20. The time required for the initially developed eggs to become fully ripe (also called the ovulation period) is usually expressed as the number of hours required at prevalent water temperatures. This is measured in degree-hours (dh).
21. During the ovulation period, you should measure the water temperature (in �C) at the end of every hour and add these values together progressively. When you approach the number of degree-hours required (see following example), your fish are getting ready to spawn.
Example
You give the decisive Injection at 20.00 h to common carp broodstock. You measure the water temperature of the holding tank every hour as follows.
By the next morning at 6.00 h, you reach a total of 239.6 degree-hours, very close to the amount required in this particular case, 240 to 260. You should start watching your fish carefully.
22. The number of degree-hours required varies with:
- the species;
- the type of hormonal treatment given;
- the size of the female fish.
The number of degree-hours required varies with: |
23. Experience should help you to improve your estimates of the number of degree-hours required in each particular case.
Example
The number of degree-hours required under various circumstances to obtain full ripening of eggs within the optimum temperature (Table 24), may vary as follows:
- common carp, 240 to 260 dh; Chinese carps 200 to 220 dh;
- common carp, one injection only: 340 to 360 dh; two injections, 240 to 260 dh after second injection;
- common carp females, 1 to 2 kg each: 130 to 250 dh; 5 to 7 kg each, 240 to 260 dh.









